

Study comment Can you answer the following Fast track questions? If you answer the questions successfully you need only glance through the module before looking at the Subsection 5.1Module summary and the Subsection 5.2Achievements. If not, proceed directly to the Subsection 1.3Ready to study? Subsection. If so, try the following Fast track questions. Study comment Having read the introduction you may feel that you are already familiar with the material covered by this module and that you do not need to study it. Bohr’s theory is of great historical importance since it prepared the way for Werner Heisenberg (1901–1976), Erwin Schrödinger (1887–1961) and others who set down the basic principles of our current quantum theory of atomic structure in the mid-1920s. Although now known to be fundamentally flawed, just like Rutherford’s model, Bohr’s model gives real insights into atomic structure and is an excellent starting point from which to discuss more modern theories of the atom. Section 4 provides a detailed account of the model of the hydrogen atom put forward in 1913 by the Danish physicist Niels Bohr (1885–1962). With just a single electron bound to its nucleus, hydrogen is the simplest of atoms and represents a natural starting point for efforts to understand the structure of atoms in general. Section 3 concentrates on the simplest atomic spectrum – that of the hydrogen atom. It introduces the concept of an atomic spectrum and defines some of the main kinds of spectra that can be observed.


Under appropriate conditions, atoms can emit or absorb light Section 2 describes the way in which the light emitted or absorbed by any particular type of atom can be used to characterize that atom and provide insight into its internal structure. This module describes the first important ‘quantum model’ of the atom and some of the evidence that supported it. The overthrow of this ‘planetary model’ of the atom – still the most advanced model that most non–physicists meet – raised the problem of understanding the internal structure of atoms to one of crucial importance and opened the way for the development of other models of atomic structure that called on ideas from the new field of quantum physics. In particular, his suggestion that each atom was like a miniature Solar System, in which the electrical force held the electrons in orbit around the nucleus in much the same way that gravitational forces bind the planets to the Sun, was soon shown to be inconsistent with the laws of physics as they were then known. The concept of the atomic nucleus quickly gained widespread acceptance, but some of Rutherford’s other ideas about the structure of atoms did not fare so well. the nuclei of helium atoms) were observed to rebound or scatter from gold atoms in thin metal foils. The presence of a nucleus at the heart of every atom was proposed in 1911 by Ernest_RutherfordErnest (later Lord) Rutherford (1871–1937) on the basis of experiments in which α–particles (i.e. The centrally located nucleus has a diameter of about 10 −14 m and occupies less than one trillionth (10 −12) of an atom’s volume, yet it contains more than 99.9% of its mass. A typical atom has a diameter of about 10 −10 m and consists of one or more negatively charged electrons moving under the electrical attraction of a small, dense, positively charged nucleus. The hundred or so different kinds of atom are the basic building blocks of all of the familiar forms of matter.
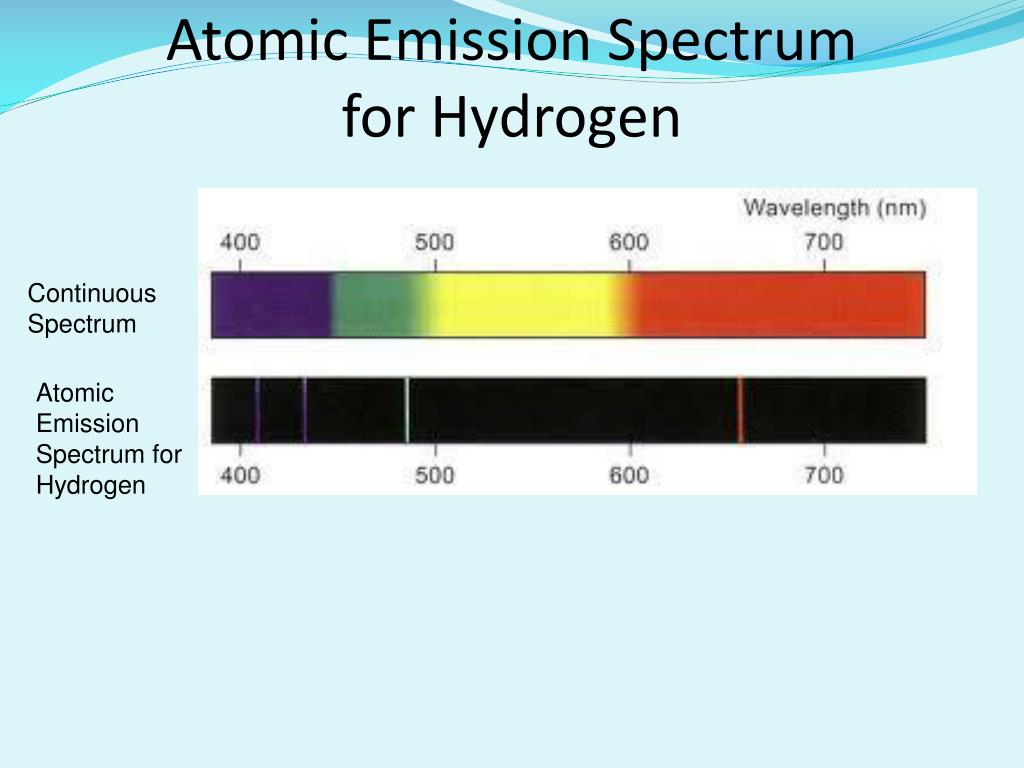
Atomic emission spectrum vs continuous spectrum series#
1 Opening items 1.1 Module introduction 1.2 Fast track questions 1.3 Ready to study? 2 The production of atomic spectra 2.1 Characteristic emission spectra 2.2 Continuous emission spectra the black–body spectrum 2.3 Absorption spectra 2.4 Summary of Section 2 3 The emission spectrum of atomic hydrogen 3.1 The visible spectrum of atomic hydrogen Balmer’s formula 3.2 The ultraviolet and infrared series of spectral lines for hydrogen 3.3 Summary of Section 3 4 Bohr’s model for the hydrogen atom 4.1 The four postulates 4.2 Derivation of the allowed orbital radii 4.3 Derivation of the electron’s orbital speed 4.4 Derivation of the kinetic, potential and total energies of an electron 4.5 The energy level diagram for atomic hydrogen unbound states and ionization 4.6 Transitions, spectral lines and Balmer’s formula 4.7 Bohr’s model for the other series in the hydrogen spectrum 5 Production of spectra excitation by heating and by collisions 6 Closing items 6.1 Module summary 6.2 Achievements 6.3 Exit test


 0 kommentar(er)
0 kommentar(er)
